Stem cell therapy is no longer experimental—it is a rapidly maturing medical field. But as clinics multiply worldwide, a critical question often goes unasked: Where do the stem cells come from, who grows them, and how good are they when they reach your bloodstream?
The answers vary enormously. In many countries, clinics offer cheap, off-the-shelf stem cells harvested from donor umbilical cords or placentas. These are convenient for the clinic—but not necessarily ideal for you. At Cell Grand Clinic in Osaka, Japan, we take a fundamentally different approach: we use your own adipose-derived (fat-derived) stem cells, cultured to pharmaceutical-grade standards in one of Japan’s most advanced academic-linked laboratories, and administered at doses of up to 200 million cells per session.
This page explains why this distinction matters—and why patients who do their homework choose Japan, choose autologous therapy, and choose Cell Grand Clinic.
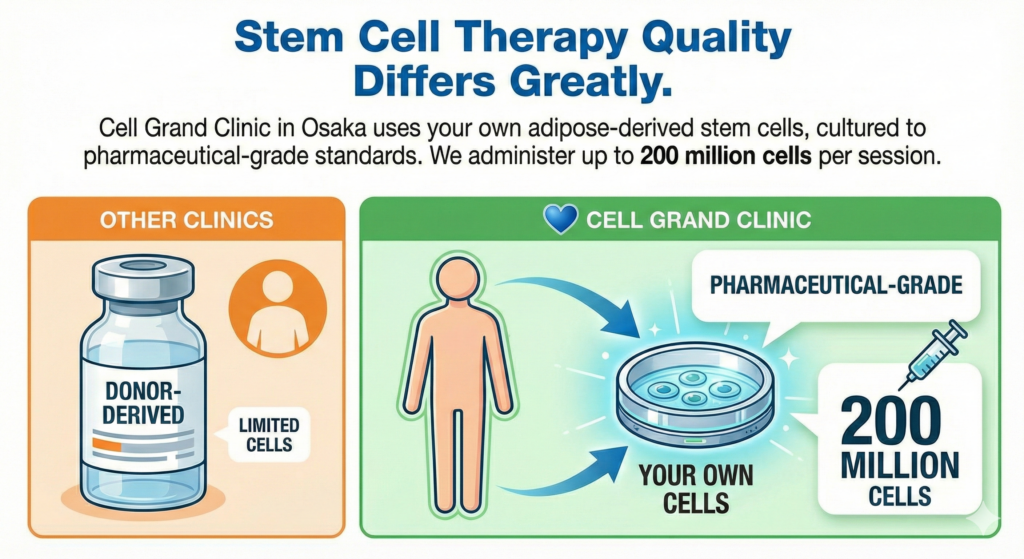
Why Autologous Adipose-Derived Stem Cells?
The Gold Standard in Regenerative Cell Therapy
Mesenchymal stem cells (MSCs) can be sourced from several tissues—bone marrow, umbilical cord, dental pulp, and adipose (fat) tissue. Among these, adipose-derived MSCs (ADMSCs) are increasingly recognized by the scientific community as the optimal source for therapeutic use. Here is why:
1. Abundance: 100–500× Greater Cell Yield Than Bone Marrow
Adipose tissue contains 100 to 500 times more mesenchymal stem cells per gram compared to bone marrow. This extraordinary abundance means we can harvest sufficient cells from just a small amount of fat—typically through a painless mini-liposuction under local anesthesia—and then expand them to therapeutic doses without over-stressing the cells during culture.
2. Superior Biological Performance
Published comparative studies demonstrate that ADMSCs outperform bone marrow-derived MSCs in multiple key areas:
- Higher proliferation capacity during culture expansion
- Greater genetic stability during long-term cultivation
- Lower immunogenicity —reduced risk of immune reactions
- Stronger immunomodulatory effects —more powerful anti-inflammatory action
3. Minimally Invasive Harvesting
Unlike bone marrow aspiration—which requires needle insertion into the hip bone, often under general anesthesia—adipose tissue is collected via a simple tiny fat harvest procedure that takes about 30 minutes under local anesthesia. Most patients return to their hotel the same day with minimal discomfort.
4. Zero Ethical Concerns
Because we use your own cells (autologous therapy), there are no ethical issues associated with embryonic or donor-derived stem cells. Your body recognizes these cells as “self,” virtually eliminating the risk of immune rejection.
Autologous vs. Allogeneic — The Critical Difference
Why “Cheaper” Is Not “Better”
Many overseas clinics promote umbilical cord-derived stem cells (UC-MSCs) from donors. These allogeneic (other-person) cells are often marketed as a cost-effective alternative. But what are you actually getting?
| Factor | Autologous ADMSCs (Cell Grand) | Allogeneic UC-MSCs (Overseas) |
| Cell Source | Your own fat tissue | Donor umbilical cord |
| Immune Rejection Risk | Virtually zero | Present — foreign cells may trigger immune response |
| Regulatory Oversight | Japan MHLW Type II license; govt-inspected CPC | Varies widely; many countries have minimal regulation |
| Cell Quality Testing | ≥98% viability; multi-marker purity testing (CD73/90/105) | Often unverified; no standardized testing required |
| Cell Passage | Low passage (P3) — maximum regenerative potency | Often high passage (P5+) — diminished therapeutic effect |
| Traceability | Full chain of custody; your cells only | Unknown donor history; pooled batches possible |
| Dose | Up to 200 million cells per session | Varies; often lower and unverified |
| Disease Transmission Risk | None — your own cells | Possible if donor screening is inadequate |
Addressing the Counter-Argument: “But UC-MSCs Are Younger and More Potent”
Proponents of allogeneic umbilical cord stem cells argue that these cells are “younger” and therefore more potent. There is a kernel of truth here: UC-MSCs do have a slightly higher initial proliferation rate. However, this advantage is largely theoretical in clinical practice for several reasons:
- Passage number matters more than source age. A UC-MSC expanded to passage 6 or 7 (common in mass-production) loses much of its therapeutic potency. Our ADMSCs at passage 3 retain far greater regenerative capacity than over-expanded UC-MSCs.
- Immune compatibility is non-negotiable. No matter how “young” a donor cell is, if your immune system attacks it, the therapeutic benefit is compromised. Autologous cells bypass this problem entirely.
- Quality control is the real differentiator. A well-cultured, rigorously tested ADMSC at 98%+ viability will outperform a poorly handled UC-MSC every time. The lab matters as much as the source.
Why Japan? The World’s Most Rigorous Regulatory Framework
When you are putting living cells into your body, the country where it happens matters enormously. Japan is the only major nation with comprehensive legislation specifically designed for regenerative medicine: the Act on the Safety of Regenerative Medicine, enacted in 2014.
What Japan’s Framework Requires
- Government-approved treatment plans reviewed by certified regenerative medicine committees before any patient is treated
- Licensed Cell Processing Centers (CPCs) meeting pharmaceutical-grade GMP standards, subject to regular government inspections
- Mandatory adverse event reporting to a national database—every complication is tracked and analyzed
- Ongoing safety surveillance with periodic inspections to ensure continued compliance
- Classification system (Type I–III) that categorizes treatments by risk level, with corresponding oversight
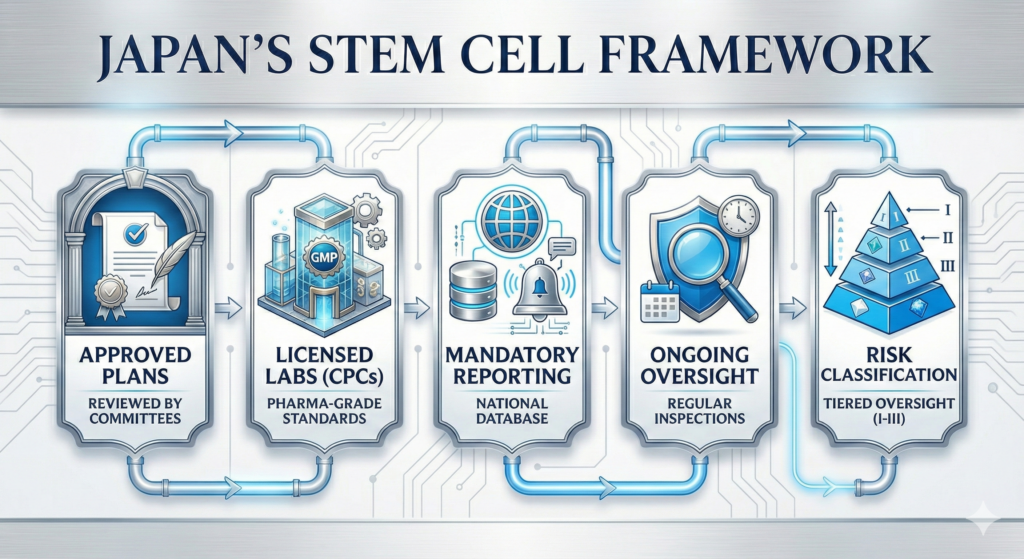
In contrast, many popular stem cell tourism destinations operate in regulatory gray zones. Clinics in some countries can administer stem cells with virtually no government oversight, no mandatory quality testing, and no adverse event tracking. The lower price often reflects the absence of these safeguards—not greater efficiency.
Cell Grand Clinic’s Government Certifications
MHLW Authorization: Ministry of Health, Labour and Welfare approved treatment protocols
Regenerative Medicine Classification: Class II (highest category for outpatient stem cell procedures)
10 Type II Government Licenses for regenerative medicine treatments
CPC Certification: Government-inspected cell processing facility (FA5250001)
Why Cell Grand Clinic?: Where Science, Quality, and Patient Care Converge
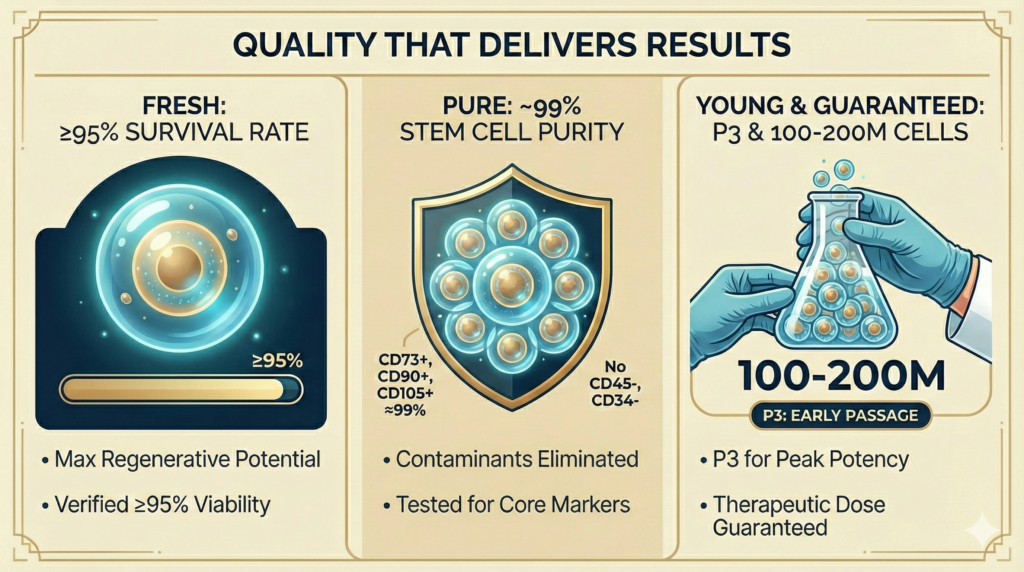
The “Fresh, Pure, Young” Cell Quality Standard
Every stem cell treatment at Cell Grand Clinic adheres to three non-negotiable quality pillars:
| Quality Pillar | Our Standard | Why It Matters |
| FRESH | 95%+ cell survival rate | Higher survival rate = greater regenerative and repair potential. Dead or dying cells cannot heal. Cell Viability ≥95% (verified before every administration) vs. industry average of 70–85% |
| PURE | ~99% stem cell surface antigen expression | CD73+, CD90+, CD105+ expression ≈99%; CD45−, CD34− (contaminants eliminated) vs. many clinics that do not test |
| YOUNG | Passage 3 cultivation; never over-expanded | Early-passage cells maintain maximum differentiation ability and biological activity. Over-passaged cells lose potency. Guaranteed 200 million cells at P3 vs. competitors using P5+ with reduced potency |
| GUARANTEED DOSE | 100 to 200 million cells per administration guaranteed | Therapeutic dosing matters. Insufficient cell counts mean insufficient results. |
Dr. Yuichi Wakabayashi — Medical Director
Cell Grand Clinic is led by Dr. Yuichi Wakabayashi, M.D., Ph.D., a physician-scientist whose credentials bridge East and West:
- NIH Research Fellow: Conducted advanced research at the U.S. National Institutes of Health
- Pfizer Collaboration: First author on the world’s first human use of a PDE4B-specific PET tracer (Journal of Nuclear Medicine, 2022)
- Board Certifications: Diplomate of the American Board of Regenerative Medicine (ABRM); multiple specialty certifications
- Published Researcher: First-author publications in Journal of Nuclear Medicine, ACS Chemical Neuroscience, and other leading journals
- Bestselling Author: Multiple Amazon #1 bestselling medical books
- Media Recognition: Featured in The Wall Street Journal as a “Next Era Leader”
- Bilingual Care: Fluent in English; 3+ years of clinical and research experience in the United States
Dr. Wakabayashi is not a business person who opened a clinic—he is a physician-scientist who has conducted cutting-edge research at the highest levels of international medicine and now applies that expertise directly to patient care.
Inside the Laboratory: Where Your Cells Come to Life
The quality of stem cell therapy is only as good as the laboratory that cultivates the cells. That’s why CELL GRAND CLINIC partners exclusively with MHLW-licensed Cell Processing Facility (CPF) — a purpose-built, pharmaceutical-grade laboratory that meets the same standards used to manufacture injectable drugs.
| FACILITY CREDENTIAL MHLW Certified Cell Processing Facility License No. FA5250001 | GMP-Grade Cleanroom | 24/7 Monitoring | Robotics-Assisted Cultivation |

World-Class Cell Processing: Academic-Linked Laboratory
Your stem cells are cultured at a government-certified Cell Processing Center (Facility No. FA5250001) that conducts joint research with Professor Takahiro Ochiya of Tokyo Medical University. Professor Ochiya is an internationally recognized authority in exosome and extracellular vesicle research:
- Ranked among the world’s leading researchers in the exosome field
- Selected as a Web of Science Highly Cited Researcher for 6 consecutive years (top 0.1% globally)
- Recipient of the ISEV Special Achievement Award (2019)
- Recipient of the 49th Princess Takamatsu Cancer Research Prize
- Over 800 peer-reviewed publications; 57,000+ citations
This is not a generic commercial lab. The scientific insights from Professor Ochiya’s world-leading research are directly applied to the cell cultivation protocols used for your treatment. This academic linkage is extremely rare in the regenerative medicine clinic space and represents a genuine quality differentiator.
Pharmaceutical-Grade Cleanroom Environment
Your cells are cultivated inside a GMP Grade A cleanroom — the highest classification in pharmaceutical manufacturing. This is the same air purity standard required for manufacturing injectable drugs and performing aseptic operations.
The facility is divided into four controlled zones (Grade A through D), each with escalating access restrictions, gowning protocols, and contamination controls:
| Grade | Purpose | Air Purity (≥0.5µm particles/m³) |
| Grade A | Stem cell culture & aseptic operations | ≤ 3,520 (strictest possible) |
| Grade B | Background environment for Grade A | ≤ 352,000 operational |
| Grade C | Preparation & non-sterile processing | ≤ 3,520,000 operational |
| Grade D | Material storage & gowning areas | Controlled per facility protocol |
Every parameter — temperature, humidity, air pressure differentials, and particle counts — is monitored 24 hours a day, 7 days a week in real time, with continuous digital recording for full regulatory traceability.
Dedicated, Certified Cell Culture Specialists
Cell cultivation is performed exclusively by trained, full-time cell culture specialists — not rotating technicians or outsourced staff. Every team member undergoes:
- Aseptic technique certification and ongoing proficiency testing
- Standard Operating Procedure (SOP) training for every step of the cultivation process
- Equipment handling and maintenance protocols
- Manufacturing record documentation meeting pharmaceutical-level audit standards
- Regulatory compliance education including Japan’s Regenerative Medicine Safety Act
- Simulation-based practical training before handling live patient cells
This is not a mass-production facility. Each patient’s cells receive individualized attention from specialists who understand that they are cultivating living therapeutic material destined for human administration.
End-to-End Traceability & Quality Control
From the moment your fat tissue arrives at the laboratory to the moment your cultured stem cells are delivered for administration, every step is documented, tested, and traceable:
- Quality control testing at each processing stage — viability counts, sterility checks, and identity confirmation
- Comprehensive manufacturing records — every parameter, every operator action, every environmental reading logged
- Chain-of-custody management — cell receipt, processing, and dispatch tracked under a unified system to eliminate mix-up or contamination risk
If any deviation occurs at any stage, the system flags it immediately for investigation — ensuring that only cells meeting the highest safety and purity standards ever reach the patient.
Next-Generation Robotics-Assisted Cell Culture
Our CPC is actively developing robotics-assisted cell cultivation technology to further enhance consistency, reproducibility, and scalability. By reducing human variability in repetitive culture steps, robotic systems deliver:
- Greater batch-to-batch consistency — ensuring every patient receives cells of uniform quality
- Reduced contamination risk — fewer human interventions mean fewer opportunities for error
- Enhanced data capture — automated systems record processing data with greater precision than manual methods
This investment in automation is not about replacing human expertise — it is about augmenting it with precision engineering to deliver the best possible therapeutic product to every patient.

The Cell Grand Clinic Experience
Located in Osaka’s prestigious Shinsaibashi district (inside the FENDI Building on Midosuji Boulevard), Cell Grand Clinic offers a patient experience unlike any other medical facility:
- Private Japanese-Modern Rooms: Every consultation and treatment takes place in a fully private, beautifully designed space inspired by high-end Japanese ryokan (traditional inns)
- Bilingual Support: English-speaking staff ensure clear communication from your first inquiry through post-treatment follow-up
- Concierge-Level Service: We assist with appointment scheduling, local recommendations, and coordination with your home physician
- Complete Privacy: No crowded waiting rooms; each patient receives individualized, unhurried attention
Our philosophy: Regenerative medicine is not just a procedure—it is an investment in your future health. You deserve an environment where you feel cared for, informed, and confident at every step.
Begin Your Journey
If you are considering stem cell therapy, we invite you to schedule a complimentary online consultation with Dr. Wakabayashi. There is no obligation—only a conversation about what regenerative medicine can realistically offer for your specific situation.
Important Disclaimers
Regenerative medicine treatments including stem cell therapy are not guaranteed to produce specific results. Individual outcomes vary based on medical history, age, condition severity, and other factors. All treatments at Cell Grand Clinic are conducted under government-approved protocols and comply with Japan’s Act on the Safety of Regenerative Medicine. The information provided on this page is for educational purposes and does not constitute medical advice. Please consult with a qualified physician to determine if stem cell therapy is appropriate for your condition.




