How 100 million adipose-derived stem cells are helping patients worldwide avoid knee replacement
- Your Knee Pain Has a Third Option — Beyond Pills and Surgery
- What’s Happening Inside Your Knee — and Why It Gets Worse
- Where Are You on the Scale? The Kellgren-Lawrence Grading System
How Stem Cell Therapy Works: The Science of Regeneration- Why Adipose-Derived Stem Cells Outperform Other Sources
- The Critical Importance of Cell Dosage: Why 100 Million Cells Matter
- Comparison: Stem Cell Therapy vs. Surgery vs. Conventional Treatments
- Why Cell Grand Clinic? What Sets Our Treatment Apart
- Treatment Process for International Patients
- Ideal Candidates for Stem Cell Therapy
- Frequently Asked Questions
- Conclusion: Restore Your Mobility—Naturally
- References
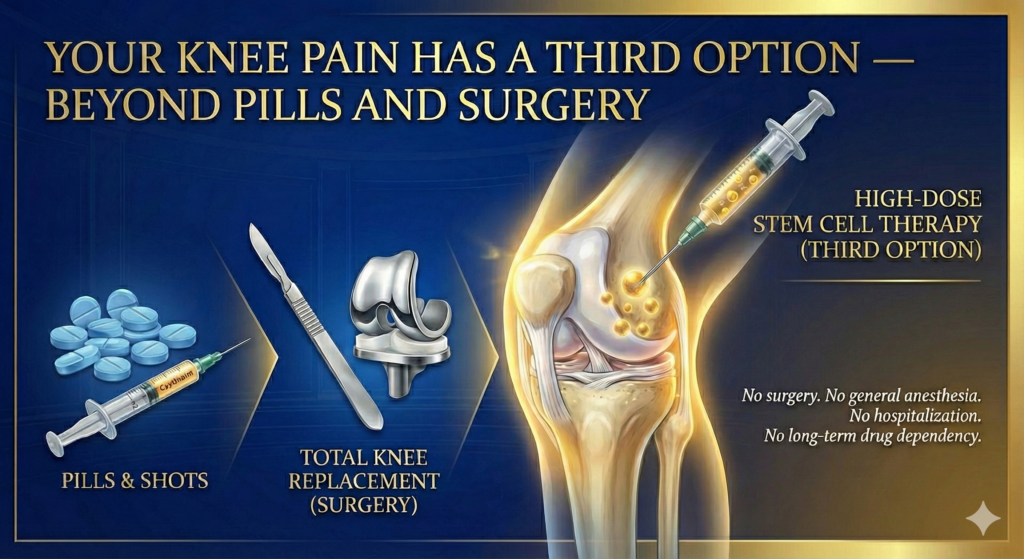
Your Knee Pain Has a Third Option — Beyond Pills and Surgery
Picture this. You’re in your 50s or 60s. You still want to play golf, hike mountain trails, or simply walk. But every step reminds you that the cartilage in your knee is disappearing.
Your doctor back home has given you two choices: keep taking anti-inflammatory drugs and cortisone shots that mask the pain while the joint deteriorates — or schedule a total knee replacement when you can’t take it anymore.
Sound familiar? You’re not alone. Knee osteoarthritis (OA) affects roughly 650 million people aged 40 and older worldwide, and those numbers are climbing every year. It is one of the leading causes of disability on the planet.
But here’s what your orthopedic surgeon may not have told you: there is now a scientifically validated third option.
Regenerative medicine — specifically, high-dose stem cell therapy — is rewriting the playbook for knee osteoarthritis treatment. And in 2025, one of the most rigorous meta-analyses ever conducted on the subject confirmed what forward-thinking physicians have long observed: injecting mesenchymal stem cells (MSCs) directly into the knee, without any surgery, can significantly reduce pain and restore joint function.
At Cell Grand Clinic in Osaka, Japan, we deliver exactly 100 million autologous adipose-derived stem cells into your knee joint — the precise high-dose protocol that the 2025 research identified as most effective. We do this under Japan’s Ministry of Health, Labour and Welfare (MHLW) oversight, one of the world’s strictest regulatory frameworks for regenerative medicine.
No surgery. No general anesthesia. No hospitalization. No long-term drug dependency.
What Does the Science Say? The 2025 Meta-Analysis Breakthrough
In March 2025, Cao et al. published a comprehensive systematic review in Stem Cell Research & Therapy, analyzing 8 randomized controlled trials (RCTs) involving 502 patients [2]. This rigorous study focused exclusively on intra-articular MSC injection without surgical intervention—providing the clearest evidence yet for stem cell efficacy in knee OA.
Key Findings
| Outcome Measure | 6-Month Results | 12-Month Results |
|---|---|---|
| WOMAC Total Score | Significant improvement (P=0.01) | Significant improvement (P=0.03) |
| Pain (VAS) | 19.39-point reduction (P=0.0008) | 16.21-point reduction (P=0.0003) |
| KOOS Subscores | Improved across all domains | Sustained improvement |
| Adverse Events | No significant difference vs. control | Safe profile maintained |
The meta-analysis concluded: “Intra-articular injection of MSCs alone could significantly improve knee pain and dysfunction in patients with unoperated OA” .
➤ Schedule Your Consultation with Dr. Wakabayashi
What’s Happening Inside Your Knee — and Why It Gets Worse
Think of knee cartilage as a precision-engineered shock absorber. In a healthy joint, this smooth tissue lets the bones in your knee glide against each other with almost zero friction. It distributes your body weight evenly and protects the underlying bone from impact.
In osteoarthritis, this cartilage breaks down. Microscopic cracks appear on the surface, deepen over time, and eventually, pieces break off entirely. When enough cartilage is lost, bone grinds directly against bone. That’s where the intense pain, swelling, stiffness, and grinding sensation (crepitus) come from.
The critical problem: adult cartilage cannot repair itself effectively. Unlike skin or bone, cartilage has no blood supply. Without blood flow, the body cannot deliver repair cells. Once cartilage damage begins, it tends to accelerate — creating a vicious cycle of inflammation, further loss, and escalating pain.
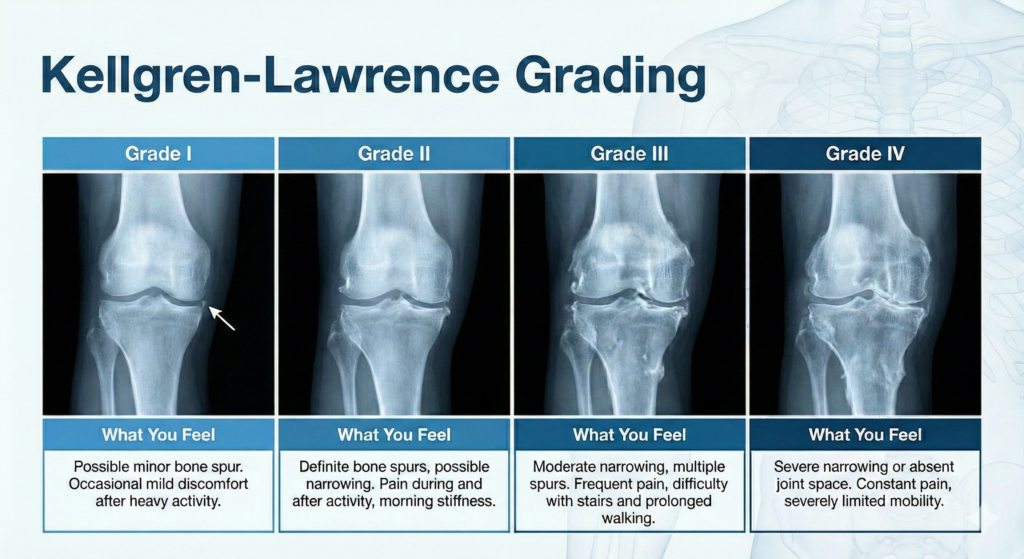
Where Are You on the Scale? The Kellgren-Lawrence Grading System
Doctors classify knee OA severity using the Kellgren-Lawrence (KL) system based on X-ray findings:
| Grade | X-Ray Findings | What You Feel |
| Grade 0 | Normal joint | No symptoms |
| Grade I | Possible minor bone spur | Occasional mild discomfort after heavy activity |
| Grade II | Definite bone spurs, possible narrowing | Pain during and after activity, morning stiffness |
| Grade III | Moderate narrowing, multiple spurs | Frequent pain, difficulty with stairs and prolonged walking |
| Grade IV | Severe narrowing or absent joint space | Constant pain, severely limited mobility |
Stem cell therapy achieves the best outcomes for patients with KL Grade II–III OA, where sufficient cartilage remains for the stem cells to support repair. Even some Grade IV patients experience meaningful pain relief.
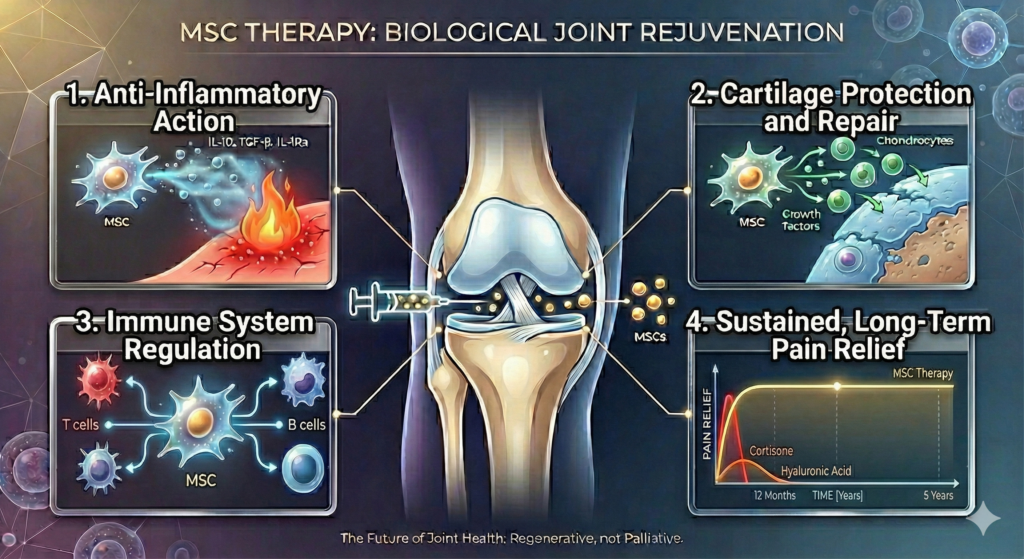
How Stem Cell Therapy Works: The Science of Regeneration
Unlike hyaluronic acid (which merely lubricates) or corticosteroids (which suppress inflammation temporarily), mesenchymal stem cells initiate genuine biological repair through multiple mechanisms:
1. Anti-Inflammatory Action
Osteoarthritis involves chronic inflammation that actively destroys cartilage. MSCs secrete powerful anti-inflammatory molecules (IL-10, TGF-β, IL-1Ra) that work like internal fire extinguishers — they don’t just reduce today’s inflammation; they break the cycle causing ongoing damage.
2. Cartilage Protection and Repair
MSCs release growth factors that stimulate chondrocytes — the cells responsible for building and maintaining cartilage. Studies have documented measurable improvements in cartilage volume and quality following high-dose MSC injection.
3. Immune System Regulation
Through sophisticated paracrine signaling, MSCs regulate T cells, B cells, and macrophages within the joint [10]. This creates a microenvironment that favors tissue repair over destruction.
4. Sustained, Long-Term Pain Relief
By addressing inflammation, improving lubrication, and supporting tissue repair simultaneously, MSCs deliver pain relief that extends far beyond conventional injections. Clinical studies have documented sustained improvements at 12 months, 24 months, and up to 5 years post-treatment.
This is fundamentally different from a cortisone shot (wears off in weeks) or hyaluronic acid (lasts a few months at best). Stem cell therapy targets the root biological causes of your pain — not just the symptoms.
Why Adipose-Derived Stem Cells Outperform Other Sources
Not all stem cells are created equal. The 2025 meta-analysis conducted subgroup analyses comparing different cell sources—and the results were striking:
Adipose-Derived MSCs (ADMSCs) vs. Bone Marrow MSCs (BM-MSCs)
| Outcome | ADMSCs | BM-MSCs |
|---|---|---|
| 6-Month WOMAC | Significant improvement (P<0.00001) | No significant improvement (P=0.27) |
| 12-Month WOMAC | Significant improvement (P<0.0001) | No significant improvement (P=0.21) |
Why adipose tissue wins:
- Higher cell yield: Abdominal fat provides significantly more stem cells than bone marrow, especially critical in elderly patients
- Easier harvesting: Liposuction is minimally invasive compared to bone marrow aspiration from the iliac crest
- Superior proliferation: Adipose-derived cells demonstrate robust expansion during culture
- Lower donor-site morbidity: No risk of prolonged pain at the harvest site
At Cell Grand Clinic, we exclusively use autologous adipose-derived stem cells harvested from your own abdominal subcutaneous fat—leveraging the most effective cell source identified in clinical research.
The Critical Importance of Cell Dosage: Why 100 Million Cells Matter
Perhaps the most clinically actionable finding from the 2025 meta-analysis was the dose-response relationship:
High-Dose (1×10⁸ cells) vs. Low-Dose (1-6.4×10⁷ cells)
| Time Point | High-Dose Group | Low-Dose Group |
|---|---|---|
| 6-Month WOMAC | Significant improvement (P=0.002) | No significant difference (P=0.16) |
| 12-Month WOMAC | Significant improvement (P<0.0001) | Marginal improvement (P=0.03) |
The largest RCT included in the meta-analysis—Kim et al. (2023) with 252 patients—used 1×10⁸ (100 million) adipose-derived stem cells and demonstrated significant efficacy in patients with Kellgren-Lawrence grade 3 knee OA.
This is precisely why Cell Grand Clinic delivers 100 million cells per treatment—meeting the threshold that research has identified for optimal therapeutic benefit.
Comparison: Stem Cell Therapy vs. Surgery vs. Conventional Treatments
| Feature | Stem Cell Therapy | Hyaluronic Acid | Knee Replacement |
| Primary Goal | Regeneration & Repair | Temporary Lubrication | Artificial Replacement |
| Invasiveness | Injection only | Injection | Major surgery |
| Recovery Time | Walk same day | None | 3-6 months rehabilitation |
| Duration of Effect | Years | Weeks to months | 15-20 years (prosthesis life) |
| Natural Joint | Preserved | Preserved | Removed |
| Serious Risks | Minimal (autologous cells) | Low | Infection, blood clots, implant failure |
Why Cell Grand Clinic? What Sets Our Treatment Apart
Quality That Delivers Results
Many clinics advertise “same-day” stem cell treatments — they harvest cells and reinject them within hours. While convenient, the cell count is far too low to reach the therapeutic threshold identified by research. Some deliver fewer than 1 million actual stem cells. Ours deliver 100 million.
Stem cells are living organisms. Like any biological product, quality varies enormously. A “stem cell treatment” at one clinic can be fundamentally different from another based on how the cells are cultivated, stored, and delivered.
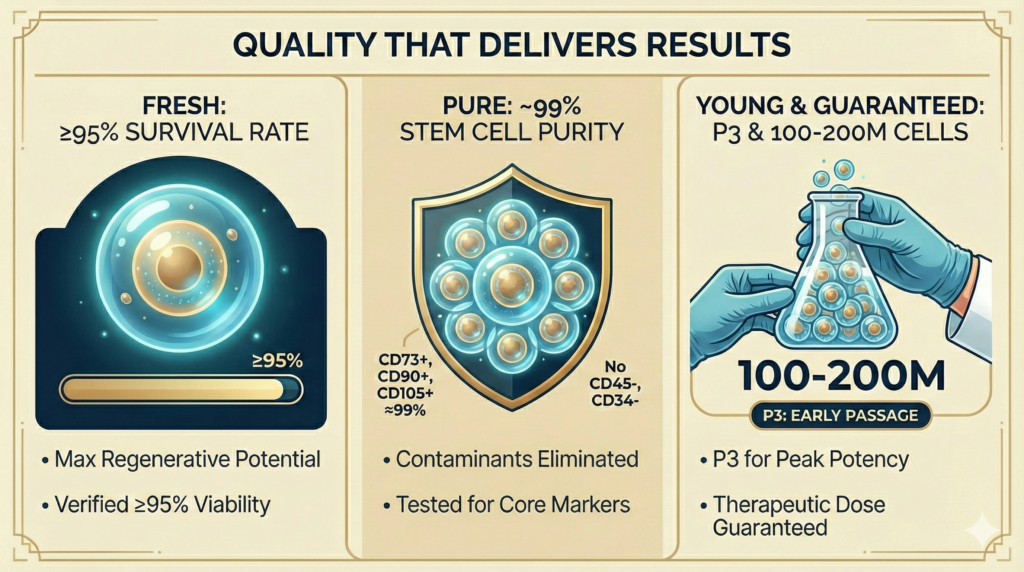
Our quality commitment: Fresh. Pure. Young.
| Quality Pillar | Our Standard | Why It Matters |
| FRESH | 95%+ cell viability | Higher viability = greater regenerative and repair potential. Dead or dying cells cannot heal. Cell Viability ≥98% (verified before every administration) vs. industry average of 70–85% |
| PURE | ~99% stem cell surface antigen expression | CD73+, CD90+, CD105+ expression ≈99%; CD45−, CD34− (contaminants eliminated) vs. many clinics that do not test |
| YOUNG | Passage 3 cultivation; never over-expanded | Early-passage cells maintain maximum differentiation ability and biological activity. Over-passaged cells lose potency. Guaranteed 100 million cells at P3 vs. competitors using P5+ with reduced potency |
| GUARANTEED DOSE | 100 million cells per administration guaranteed | Therapeutic dosing matters. Insufficient cell counts mean insufficient results. |
All cells are processed at a GMP-compliant Cell Processing Center (CPC) certified under Japanese pharmaceutical standards — the same level of quality control applied to manufactured drugs.
Japanese Government Approval: The World’s Gold Standard
Japan’s Act on the Safety of Regenerative Medicine (2014, regularly updated) created one of the world’s first comprehensive legal frameworks for stem cell treatments. Clinics must submit detailed treatment plans to MHLW-certified committees for review and approval.
13 certified treatment plans (Type II and III licenses) —
Cell Grand Clinic holds One of the broadest portfolios of any regenerative medicine clinic in Japan. Our knee OA treatment is not “experimental” or “off-label.” It is a government-regulated medical treatment.
Compare this to the situation in many other countries, where stem cell clinics operate with little regulatory oversight, using uncharacterized cell preparations with no quality control requirements.
For more information about stem cells, click here.
Academic Partnership and Expert Oversight
Our cell culture protocols are developed in collaboration with Professor Takahiro Ochiya’s research team, whose work on exosomes and stem cell biology has appeared in top-tier journals. Every treatment plan is personally overseen by Dr. Yuichi Wakabayashi, Diplomate of the American Board of Regenerative Medicine, ensuring care that meets the highest international standards.
➤ Schedule Your Consultation with Dr. Wakabayashi
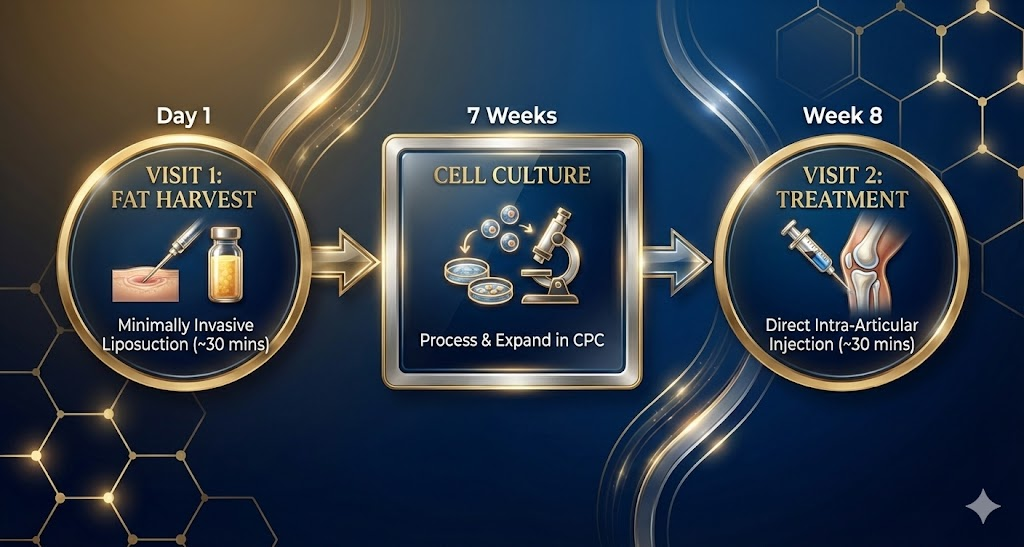
Treatment Process for International Patients
We’ve optimized our protocol for busy professionals and international visitors:
Step 1: Remote Consultation Share your MRI/X-ray results with our medical team for preliminary assessment.
Step 2: Visit 1 – Fat Harvest (Day 1) Minimally invasive fat harvest (~30 minutes) under local anesthesia. Return to your hotel immediately.
Step 3: Cell Culture (7 Weeks) Your cells are processed and expanded in our specialized CPC. Return home while we cultivate your cells to therapeutic levels.
Step 4: Visit 2 – Treatment (Week 8) Return to Osaka for stem cell administration via direct intra-articular injection (~30 minutes including preparation).
Note: Repeat treatments may be recommended depending on disease severity.
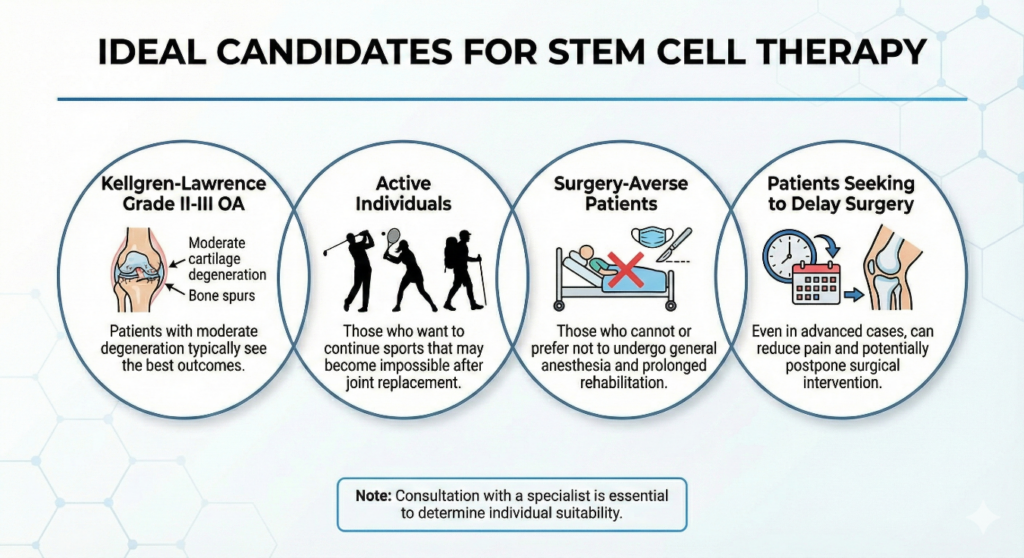
Ideal Candidates for Stem Cell Therapy
This therapy is particularly effective for:
- Kellgren-Lawrence Grade II-III OA: Patients with moderate degeneration typically see the best outcomes
- Active individuals: Those who want to continue sports (golf, tennis, hiking) that may become impossible after joint replacement
- Surgery-averse patients: Those who cannot or prefer not to undergo general anesthesia and prolonged rehabilitation
- Patients seeking to delay surgery: Even in advanced cases, stem cell therapy can reduce pain and potentially postpone surgical intervention
Frequently Asked Questions
Q: How does this compare to total knee replacement?
A: Surgery replaces your joint with artificial components; stem cell therapy aims to preserve and repair your natural joint. For appropriate candidates, stem cell therapy can reduce pain by 50-80% and significantly improve mobility, potentially eliminating or delaying the need for surgery [12].
Q: Is there an age limit?
A: No strict limit. We have successfully treated patients in their 80s. The 7-week culture protocol is especially important for older patients, as it allows us to expand even cells with reduced proliferative capacity to therapeutic numbers.
Q: Can I walk immediately after treatment?
A: Yes. Unlike surgery, no cast or crutches are required. You can walk out of the clinic. We recommend avoiding high-impact activities for several weeks to allow cellular integration.
Q: How long do results last?
A: Clinical studies have shown sustained improvements at 12 months and beyond [2,11]. Individual results vary based on OA severity, activity level, and overall health.
Q: Is this treatment safe?
A: The 2025 meta-analysis found no significant difference in adverse events between stem cell and control groups [2]. Since we use your own cells (autologous), immune rejection is not a concern. The most common side effects are temporary pain and swelling at the injection site.
Conclusion: Restore Your Mobility—Naturally
Your knees are your foundation. Don’t replace them prematurely—restore them.
With the optimal combination of: –
✓ Adipose-derived stem cells (the most effective source) –
✓ 100 million cells (the high-dose protocol) –
✓ Japanese government regulatory approval (highest safety standards) –
✓ 7-week culture process (maximum potency)
Cell Grand Clinic offers the scientifically validated, non-surgical solution for knee osteoarthritis.
➤ Schedule Your Consultation with Dr. Wakabayashi
References
This article is for informational purposes only and does not constitute medical advice. Consult with a qualified healthcare provider to determine if stem cell therapy is appropriate for your condition.
Last updated: February 2025




