How Autologous Adipose-Derived Stem Cells Are Helping International Patients Break Free from the Painkiller Cycle
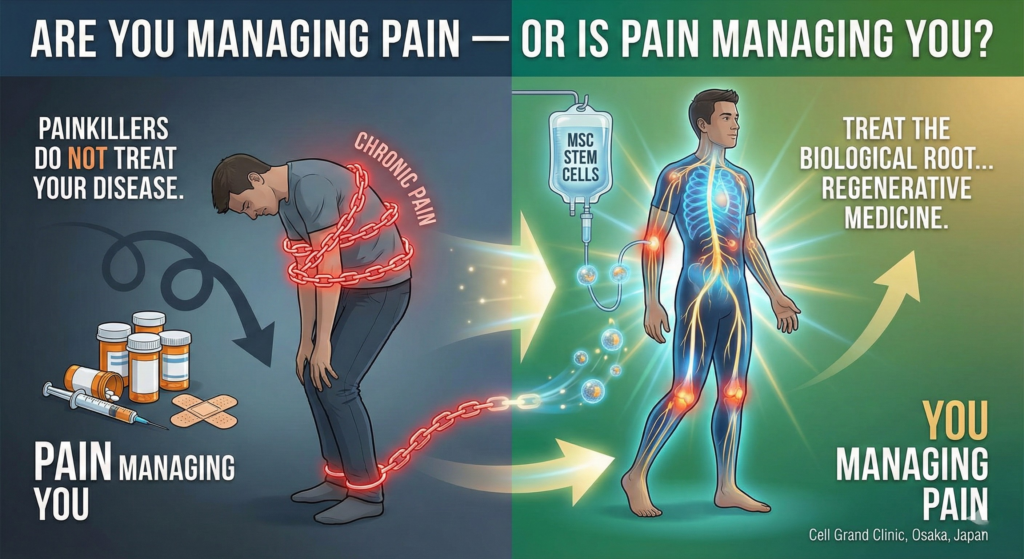
Are You Managing Pain — or Is Pain Managing You?
If you are living with chronic pain, you already know the routine. You take your medication. The edge comes off for a few hours. Then it returns — sometimes worse than before. You have tried physical therapy, steroid injections, and nerve blocks. You have adjusted your entire life around a condition that no one seems able to fix. And every time you visit a new specialist, the answer is the same: another prescription.
Whether your doctor has diagnosed you with lumbar spondylosis, a herniated disc, rheumatoid arthritis, fibromyalgia, neuropathic pain, or complex regional pain syndrome (CRPS), you already understand one truth that conventional medicine often ignores: painkillers do not treat your disease. They simply make it tolerable enough to endure another day.
The numbers tell a sobering story. According to the CDC, approximately one in five American adults lives with chronic pain. The 2018 SPACE Trial published in JAMA found that opioids were no more effective than non-opioid treatments at improving physical function over 12 months — yet they carried significantly greater risks, including dependency, cognitive impairment, and overdose. Globally, the WHO estimates that chronic pain affects more than 1.5 billion people, making it one of the most pervasive and undertreated conditions in medicine.
At Cell Grand Clinic in Osaka, Japan, we offer a fundamentally different approach. Instead of blocking pain signals, we treat the biological root of your pain — inflammation, immune dysfunction, and nerve damage — using your own stem cells. Our intravenous autologous adipose-derived stem cell therapy delivers 100 million or 200 million high-potency mesenchymal stem cells (MSCs) directly into your bloodstream, targeting damaged tissues throughout your entire body. This is not pain management. This is regenerative medicine.
Which Type of Chronic Pain Do You Have?
Chronic pain is not one disease. It is a broad category encompassing many distinct conditions, each driven by different underlying mechanisms. A treatment approach that works for osteoarthritis may be completely wrong for neuropathic pain, and the medication regimen prescribed for fibromyalgia would be inappropriate for a herniated disc. Understanding which type of pain you are experiencing is the essential first step toward finding a treatment that addresses the actual cause.
Review the descriptions below and identify the condition that best matches your experience. If you recognize yourself in more than one category, you are not unusual — many chronic pain patients live with overlapping conditions, which is another reason why a systemic, whole-body treatment approach can be more effective than targeting a single site.
| Your Condition | What You Feel | Why Painkillers Fall Short |
| Lumbar Spondylosis / Spinal Stenosis | Deep, aching low-back pain that worsens with standing or walking. Morning stiffness. Numbness or tingling radiating into the legs. Difficulty maintaining posture. | Painkillers mask the signal but cannot reverse disc degeneration, bone spur growth, or the narrowing of the spinal canal that compresses your nerves. |
| Herniated Disc (Cervical / Lumbar) | Sharp, shooting pain from the neck or back into the arms or legs. Worsens with bending, coughing, or prolonged sitting. Muscle weakness or numbness in the extremities. | Anti-inflammatory drugs reduce swelling temporarily but cannot repair the ruptured disc annulus, heal the irritated nerve root, or restore disc height. |
| Rheumatoid Arthritis | Symmetrical joint pain and swelling, especially in the hands, wrists, and knees. Morning stiffness lasting more than 30 minutes. Systemic fatigue and malaise. | Immunosuppressants and DMARDs slow disease progression but carry serious long-term side effects (infection risk, liver damage) and cannot regenerate destroyed joint tissue. |
| Neuropathic Pain (Diabetic Neuropathy, Post-Herpetic Neuralgia, CIPN) | Burning, electric-shock sensations, numbness, or pins-and-needles. Pain triggered by light touch (allodynia). Often worst at night, disrupting sleep. | Gabapentinoids and antidepressants blunt nerve signals but cannot repair damaged nerve fibers, restore myelin sheath integrity, or reverse the underlying neuropathy. |
| Fibromyalgia | Widespread musculoskeletal pain with no clear structural cause. Profound fatigue, sleep disturbance, and cognitive fog (“fibro fog”). Pain that seems to migrate. | Localized treatments miss the mark. Fibromyalgia involves central sensitization and systemic inflammation requiring a whole-body approach that single-site injections cannot provide. |
| CRPS (Complex Regional Pain Syndrome) | Severe, burning pain disproportionate to the original injury. Skin color and temperature changes. Swelling, sensitivity to touch, and movement limitation. | CRPS involves immune dysregulation and neuroinflammation that standard pain medications cannot resolve. Cleveland Clinic’s NIH-funded research is now investigating MSCs specifically for CRPS. |
| Failed Back Surgery Syndrome / Post-Surgical Pain | Persistent or worsening pain after spinal or joint surgery. Scar tissue formation restricting movement. Ongoing nerve irritation despite surgical intervention. | Further surgery often creates more scar tissue. Opioids and nerve blocks provide temporary symptom relief but do not address the fibrosis or ongoing inflammation. |
If any of these descriptions sound like your daily reality, you are not alone — and you are not out of options. Stem cell therapy targets the underlying biological mechanisms that all of these conditions share: chronic inflammation, immune dysfunction, and nerve damage.
Why Conventional Pain Management Fails — and Why This Matters
To understand why regenerative medicine represents a genuine breakthrough, you need to understand why current treatments so often fail. Chronic pain is not simply a prolonged version of acute pain. In many cases, it is a self-perpetuating malfunction of the nervous system and immune system that continues long after any original injury has healed. Researchers call this phenomenon “central sensitization” — the nervous system itself becomes rewired to amplify pain signals, even when the original trigger is no longer present.
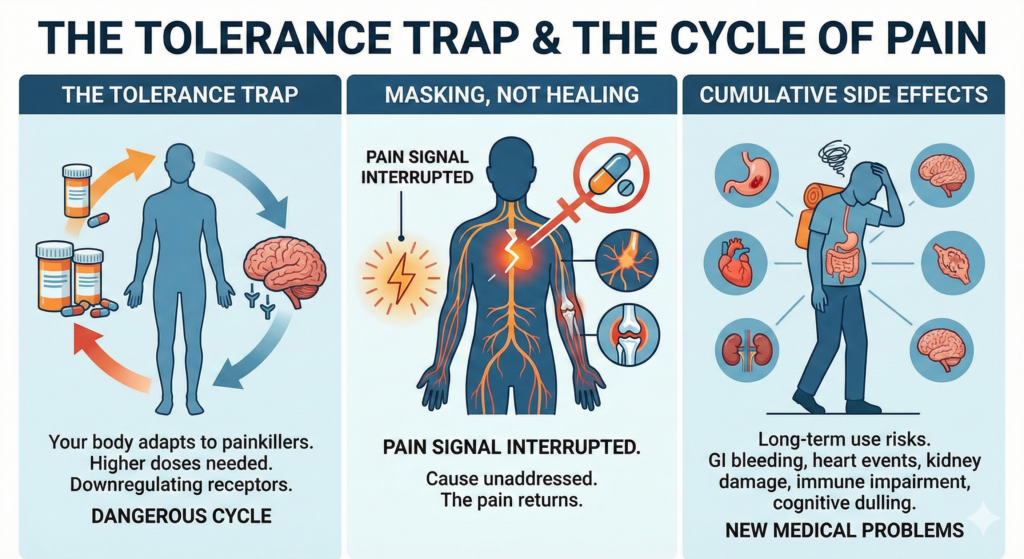
The Tolerance Trap
Your body adapts to painkillers. Over time, higher doses are needed for the same relief, creating a dangerous cycle of escalating dependency. This is not a matter of willpower — it is basic pharmacology. Opioid receptors downregulate with chronic exposure, meaning the drug becomes progressively less effective. Meanwhile, the risks of overdose, cognitive decline, and organ damage increase with every dosage escalation.
Masking, Not Healing
Every painkiller on the market works the same fundamental way: it interrupts the pain signal traveling to your brain. NSAIDs reduce prostaglandin production. Opioids bind to mu-receptors. Gabapentinoids modulate calcium channels. But none of them repair damaged nerves, regenerate deteriorated cartilage, calm chronic systemic inflammation, or correct the immune dysfunction driving rheumatoid arthritis. The moment the drug wears off, the pain returns — because the cause was never addressed.
Cumulative Side Effects
Long-term NSAID use is associated with gastrointestinal bleeding, cardiovascular events, and kidney damage. Chronic opioid use impairs immune function, disrupts hormonal balance, and increases fall risk. Gabapentinoids cause weight gain, dizziness, and cognitive dulling. These medications create new medical problems while failing to solve the original one — trapping patients in a cycle where the treatment itself becomes part of the disease burden.
This is the critical difference: Painkillers treat symptoms. Stem cell therapy treats the disease process itself — the inflammation, the immune dysregulation, and the nerve damage that generate chronic pain in the first place.
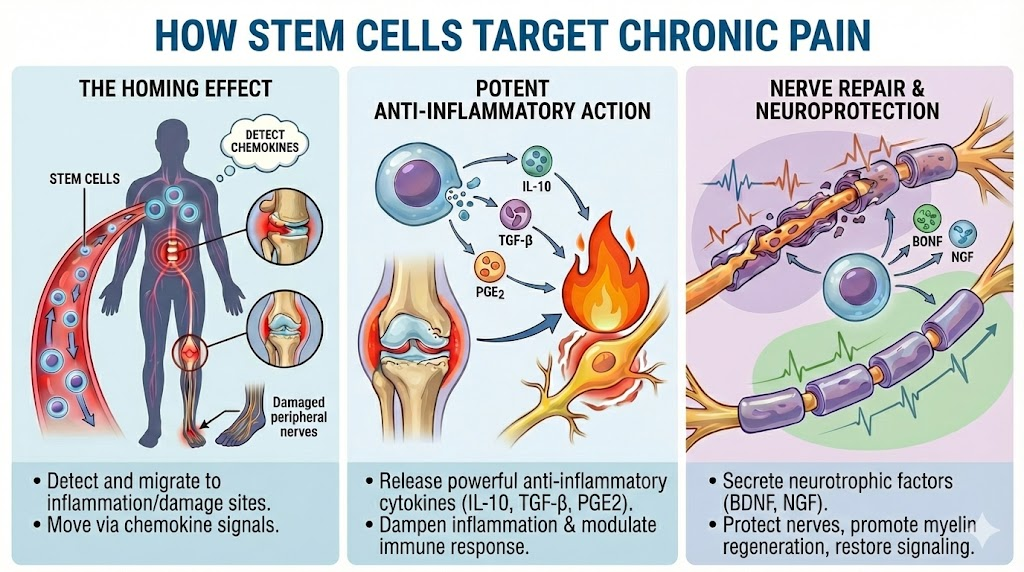
How Stem Cell Therapy Treats Chronic Pain at the Source
Regenerative medicine represents a true paradigm shift in chronic pain treatment. Instead of blocking signals, we use your own stem cells to reset the biological environment generating the pain. We administer high-potency mesenchymal stem cells (MSCs) via intravenous drip to treat your entire body systemically — not just one localized area. This systemic approach is particularly powerful for conditions like fibromyalgia, rheumatoid arthritis, and neuropathic pain, where damage and inflammation are distributed throughout the body.
The Homing Effect: Intelligent Biological Navigation
When introduced into your bloodstream, stem cells detect chemical distress signals called chemokines released by inflamed tissues and damaged nerves throughout the body. They then migrate specifically to these problem areas — whether that is a degenerated lumbar disc, arthritic joints eroded by rheumatoid disease, peripheral nerves damaged by diabetes, or the widespread neuroinflammation characteristic of fibromyalgia and CRPS. This natural homing ability is what makes intravenous delivery uniquely effective for chronic pain conditions that affect multiple body regions simultaneously.
Potent Anti-Inflammatory Action
Chronic pain is fueled by chronic inflammation. Once at the site of injury, stem cells release powerful anti-inflammatory cytokines — including IL-10, TGF-β, and prostaglandin E2 — that collectively downregulate the inflammatory cascade. For rheumatoid arthritis patients, this immunomodulatory effect is particularly significant: stem cells help recalibrate the misdirected immune system that is destroying your joint tissue, offering a fundamentally different mechanism of action from conventional immunosuppressant drugs. For patients with herniated discs and lumbar spondylosis, these anti-inflammatory properties reduce the chemical irritation of compressed nerve roots that drives much of the radiating pain.
Nerve Repair and Neuroprotection
For conditions involving nerve damage — diabetic neuropathy, post-herpetic neuralgia, chemotherapy-induced peripheral neuropathy, or the neuroinflammation seen in CRPS — stem cells secrete neurotrophic factors including BDNF (Brain-Derived Neurotrophic Factor) and NGF (Nerve Growth Factor). These proteins protect existing nerves from further degeneration, promote regeneration of the myelin sheath essential for proper signal transmission, and help rewire abnormal pain signaling pathways toward a normal, non-pain state. This neuroprotective mechanism is something that no pharmaceutical painkiller can replicate.
➤ Book Your Private Consultation
Stem Cell Therapy vs. Conventional Pain Treatment: A Clear Comparison
| Factor | Painkillers / Opioids | Nerve Blocks / Steroid Injections | IV Stem Cell Therapy (Cell Grand Clinic) |
| Primary Goal | Mask pain signals | Block specific nerve pathways temporarily | Treat root cause: repair tissue, regenerate nerves, modulate immunity |
| Duration | Hours (requires daily dosing) | Weeks to months (repeated procedures) | Months to years (biological repair continues) |
| Treats Multiple Pain Sites | Partially (systemic drugs affect whole body but heal nothing) | No (one site per injection) | Yes (IV delivery reaches every affected area) |
| Dependency Risk | High (especially opioids and gabapentinoids) | Low, but diminishing returns with repetition | None (your own cells, no pharmacological dependency) |
| Side Effect Profile | Liver/kidney damage, brain fog, GI bleeding, hormonal disruption, addiction | Temporary numbness, cartilage degradation with repeated steroids, infection risk | Minimal (autologous cells; no immune rejection risk) |
| Addresses Nerve Damage | No | No | Yes (neurotrophic factor secretion promotes nerve regeneration) |
| Reverses Immune Dysfunction | No | No | Yes (immunomodulation recalibrates overactive immune response) |
Why Japan — and Why Cell Grand Clinic?
Japan’s Gold-Standard Regulatory Framework
Japan is the only major country with a dedicated national law governing regenerative medicine. Under the Act on the Safety of Regenerative Medicine (ASRM, enacted 2014), every stem cell treatment must be government-approved through a rigorous application to the Ministry of Health, Labour and Welfare (MHLW), processed in licensed pharmaceutical-grade Cell Processing Centers subject to regular government inspection, and reported to a national safety database with mandatory adverse event monitoring. This is not voluntary compliance — it is enforceable law.
Many popular stem cell tourism destinations in Southeast Asia, Central America, and Eastern Europe have no equivalent regulatory framework. The lower prices you see elsewhere often reflect the absence of these critical safeguards — not superior value. When your health is at stake, the cost of unregulated treatment can be measured in complications, not savings.
World-Class Scientific Partnership
Your stem cells are cultured at a government-certified Cell Processing Center (CPC License: FA5250001) connected to the research of Professor Takahiro Ochiya of Tokyo Medical University — a Web of Science Top 0.1% Highly Cited Researcher for six consecutive years, with over 800 peer-reviewed publications and 57,000+ citations. His pioneering exosome research directly informs the cultivation protocols used on your cells. This caliber of academic-clinical partnership is virtually unheard of in the private regenerative medicine sector anywhere in the world.
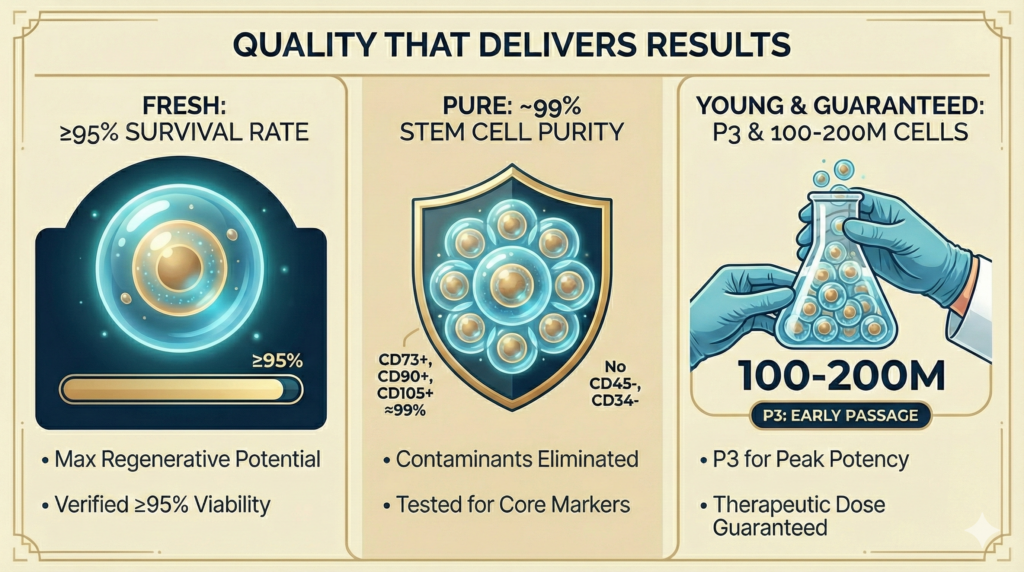
Our Cell Quality Standard: Fresh, Pure, Young
Many clinics offer a few million cells—doses too small for systemic effect. We invest 7 weeks cultivating your cells to a maximum of 200 million viable cells (2×10⁸). Every batch is over 95% survival rate, only early-passage (P3) cells for maximum potency, tested for sterility and genetic stability. Our philosophy: “Fresh, Pure, Young”—
| Quality Pillar | Our Standard | Why It Matters |
| FRESH | 95%+ cell survival rate | Higher survival rate = greater regenerative and repair potential. Dead or dying cells cannot heal. Cell Viability ≥95% (verified before every administration) vs. industry average of 70–85% |
| PURE | ~99% stem cell surface antigen expression | CD73+, CD90+, CD105+ expression ≈99%; CD45−, CD34− (contaminants eliminated) vs. many clinics that do not test |
| YOUNG | Passage 3 cultivation; never over-expanded | Early-passage cells maintain maximum differentiation ability and biological activity. Over-passaged cells lose potency. Guaranteed 100 million cells at P3 vs. competitors using P5+ with reduced potency |
| GUARANTEED DOSE | 100 to 200 million cells per administration guaranteed | Therapeutic dosing matters. Insufficient cell counts mean insufficient results. |
Your Doctor: A Physician-Scientist Who Speaks Your Language
Dr. Yuichi Wakabayashi, M.D., Ph.D. brings credentials that bridge East and West. He completed postdoctoral training at the National Institutes of Health (NIH) in the United States. He is the first author on a Pfizer-collaborated world-first PET tracer study published in the Journal of Nuclear Medicine (2022), a Diplomate of the American Board of Regenerative Medicine (ABRM), and was featured in The Wall Street Journal as a “Next Era Leader.” Critically, Dr. Wakabayashi is fluent in English, allowing direct doctor-patient communication with no interpreters needed.
Cell Grand Clinic holds 10 Type II Government Licenses — the highest outpatient classification issued by Japan’s Ministry of Health, Labour and Welfare.
For more information about stem cells, click here.

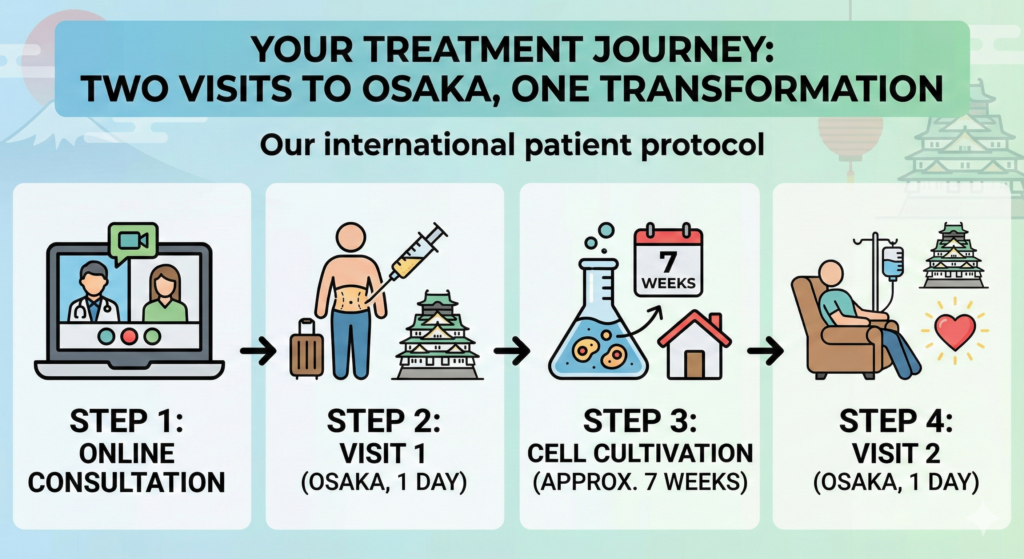
Your Treatment Journey: Two Visits to Osaka, One Transformation
We designed our international patient protocol to respect your time while delivering maximum therapeutic impact. The entire process requires only two brief visits to Osaka, Japan.
Step 1: Online Consultation (Remote)
Submit your medical history, imaging, and current medication list. Dr. Wakabayashi personally reviews your case to assess suitability and design your individualized treatment plan. All communication is conducted in English, and consultations can be arranged across international time zones.
Step 2: Fat Harvest — Visit 1 (1 Day in Osaka)
A minimally invasive, small-volume fat harvesting procedure is performed from the abdomen under local anesthesia. The procedure takes approximately 30 minutes, and you walk out immediately. There is no general anesthesia, no hospitalization, and no stitches required.
Step 3: Cell Cultivation (Approximately 7 Weeks)
You return home while our CPC team cultivates your cells to maximum potency in the government-certified laboratory. During this period, your stem cells are expanded to 100 million or 200 million cells, depending on your treatment plan. Every batch undergoes rigorous quality control testing before your return visit.
Step 4: IV Administration — Visit 2 (1 Day in Osaka)
Return to Osaka for your stem cell infusion via painless intravenous drip. Treatment takes 60 to 90 minutes in a comfortable private room. Many international patients combine this visit with travel in Osaka, Kyoto, or Nara — turning their second medical visit into an opportunity to experience Japan.
Beyond Pain Relief: The Anti-Aging Benefit
While most patients come to Cell Grand Clinic seeking relief from chronic pain, many discover an unexpected benefit: systemic rejuvenation. The same anti-inflammatory and regenerative properties that reduce chronic pain also improve vascular health, skin elasticity, energy levels, and immune function. International patients who have already invested in their health through stem cell therapy for pain often choose to continue with periodic treatments as part of a comprehensive longevity strategy. This dual benefit — treating a specific medical condition while simultaneously supporting overall healthspan — is one of the reasons wealthy, health-conscious individuals from around the world choose Cell Grand Clinic for regenerative medicine.
Frequently Asked Questions
Can I reduce or stop my pain medications after treatment?
That is the goal. While we advise against stopping medications abruptly, many patients find that as systemic inflammation subsides over the weeks and months following treatment, their need for medication naturally decreases. Some patients are able to discontinue certain medications entirely under the supervision of their prescribing physician. Your home doctor should oversee any medication changes.
Is this treatment safe?
Yes. We use autologous cells — your own cells harvested from your own body — which eliminates the risk of immune rejection. The procedure is entirely non-surgical (IV drip only), and Japan’s ASRM law ensures that every cell batch is tested for sterility, mycoplasma, endotoxins, and other contaminants before administration. Cell Grand Clinic has administered stem cell therapy under government license since its establishment, with an excellent safety record.
How soon will I feel results?
Stem cell therapy is not an instant fix like a painkiller. It works through biological processes that take time. During weeks one through four, many patients report improved sleep quality and reduced fatigue as the earliest noticeable changes. Over months one to three, pain levels gradually decrease as inflammation is suppressed and damaged tissues begin to repair. Long-term therapeutic effects can persist for a year or more, depending on the specific condition and individual response. Some patients opt for follow-up treatments to sustain and build upon their results.
Is IV stem cell therapy effective for fibromyalgia specifically?
Yes. Fibromyalgia is increasingly understood to involve systemic inflammation and central sensitization — exactly the mechanisms that IV stem cell therapy targets. Unlike localized injections or nerve blocks, systemic IV delivery reaches the entire body, addressing the widespread nature of fibromyalgia pain. This whole-body approach is one of the reasons our fibromyalgia patients report some of the most dramatic improvements.
Why can’t I get this treatment in my home country?
In many countries, including the United States, ex-vivo expansion (laboratory cultivation) of stem cells for clinical use is not permitted under current FDA regulations. Japan’s ASRM law specifically created a legal pathway for these advanced treatments while maintaining rigorous safety standards. This regulatory innovation is what makes Japan the global destination for regenerative medicine.
How does Japan compare to other stem cell tourism destinations?
Japan’s ASRM law (2014) is the only national regulatory framework dedicated specifically to regenerative medicine. This means mandatory government licensing for every clinic, mandatory processing in certified laboratories, mandatory safety reporting, and regular government audits. No other major stem cell tourism destination — including Mexico, Thailand, Panama, or Colombia — offers this level of regulatory oversight and patient protection.
What Critics Say — and Why Transparency Matters
“The evidence base is still developing.”
This is partially true. While large-scale randomized controlled trials specifically for IV MSC therapy in chronic pain are still ongoing, the body of preclinical evidence, case series, and early-phase clinical data is substantial and growing rapidly. The biological mechanisms — immunomodulation, neuroprotection, and anti-inflammatory action — are well-established across hundreds of peer-reviewed publications. The Cleveland Clinic’s $5.5 million NIH-funded study on MSCs for CRPS (2022) reflects the growing institutional recognition of this therapeutic approach. We are transparent with every patient: this is an advanced, evidence-informed treatment that has helped many patients achieve meaningful improvement, but it is not yet classified as standard-of-care.
“Some stem cell clinics make exaggerated claims.”
We agree completely. The global stem cell industry includes unregulated operators making unfounded promises — and their existence harms every legitimate practice in this field. This is precisely why we emphasize Japan’s regulatory framework, our government certifications, published cell quality metrics, and the academic credentials behind our protocols. We do not promise a cure. We offer a scientifically grounded treatment backed by Japan’s most rigorous safety standards, with a realistic pathway toward meaningful improvement in pain, function, and quality of life.
Take the First Step Toward Real Pain Relief
If you have been living with chronic pain and conventional treatments have not given you the relief you deserve, it may be time to explore a fundamentally different approach. Stem cell therapy at Cell Grand Clinic in Osaka, Japan offers a realistic pathway toward reducing your dependence on pain medication, addressing the root biological cause of your pain, and reclaiming the quality of life that chronic pain has taken from you.
Dr. Wakabayashi offers complimentary initial consultations in English for international patients. Whether your diagnosis is lumbar spondylosis, a herniated disc, rheumatoid arthritis, fibromyalgia, neuropathic pain, CRPS, or treatment-resistant chronic pain of any kind, we welcome the opportunity to review your case and discuss whether our autologous adipose-derived stem cell therapy protocol — delivering 100 million or 200 million cells via IV — is appropriate for your specific condition.
Contact us today to schedule your complimentary online consultation.
References
Medical Disclaimer
This article is for informational purposes only and does not constitute medical advice. Stem cell therapy outcomes vary by individual. All treatments at Cell Grand Clinic are performed under Japan’s Act on the Safety of Regenerative Medicine (2014) with appropriate government licensing. Consult your physician before making any changes to your current treatment plan.




